Dibenzoyl-L-tartaric acid

Get a Quick Quote Now
Select Packaging
Product
Dibenzoyl-L-tartaric acid
CAS
2743-38-6
Formula
C18H14O8
Product Description
Dibenzoyl-L-tartaric acid is a chemical compound with the formula C18H14O8, commonly used in various chemical applications. It is known for its role as a chiral auxiliary in asymmetric synthesis, aiding in the production of enantiomerically pure compounds.
Need a quote for Dibenzoyl-L-tartaric acid?
Please fill out the RFQ form, and one of our representatives will contact you as soon as possible.
Typical Product Specifications
Canonicalized Compound
1
Compound Complexity
483
Hydrogen Bond Acceptor Count
8
Hydrogen Bond Donor Count
2
Rotatable Bond Count
9
Allowed IUPAC Name
(2R,3R)-2,3-dibenzoyloxybutanedioic acid
CAS-like Style IUPAC Name
(2R,3R)-2,3-dibenzoyloxybutanedioic acid
Markup IUPAC Name
(2<I>R</I>,3<I>R</I>)-2,3-dibenzoyloxybutanedioic acid
Preferred IUPAC Name
(2R,3R)-2,3-dibenzoyloxybutanedioic acid
Systematic IUPAC Name
(2R,3R)-2,3-bis(phenylcarbonyloxy)butanedioic acid
Traditional IUPAC Name
(2R,3R)-2,3-dibenzoyloxysuccinic acid
Standard InChI
InChI=1S/C18H14O8/c19-15(20)13(25-17(23)11-7-3-1-4-8-11)14(16(21)22)26-18(24)12-9-5-2-6-10-12/h1-10,13-14H,(H,19,20)(H,21,22)/t13-,14-/m1/s1
Standard InChIKey
YONLFQNRGZXBBF-ZIAGYGMSSA-N
XLogP3-AA Log P
2.6
Exact Mass
358.06886740
Molecular Weight
358.3
Canonical SMILES
C1=CC=C(C=C1)C(=O)OC(C(C(=O)O)OC(=O)C2=CC=CC=C2)C(=O)O
Isomeric SMILES
C1=CC=C(C=C1)C(=O)O[C@H]([C@H](C(=O)O)OC(=O)C2=CC=CC=C2)C(=O)O
Polar Surface Area Topological
127
MonoIsotopic Weight
358.06886740
Chemical Structure
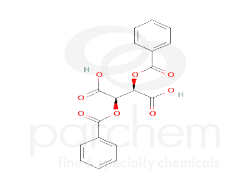
Class
Uses of Dibenzoyl-L-tartaric acid
In the pharmaceutical and chemical industries, Dibenzoyl-L-tartaric acid is primarily utilized as a chiral auxiliary in the synthesis of pharmaceuticals and other fine chemicals. Its ability to influence the stereochemistry of reactions makes it valuable in the production of drugs that require specific enantiomeric forms.
Industries that use Dibenzoyl-L-tartaric acid
- Pharmaceutical - Used in the synthesis of chiral drugs, ensuring the production of the desired enantiomer for therapeutic efficacy.
- Chemical Manufacturing - Serves as a reagent in various chemical reactions, particularly in the production of fine chemicals and intermediates.
- Research and Development - Employed in laboratories for the development of new synthetic methodologies and in the study of stereochemistry.
Related Products
- Complementary Products
- Alternative Products
Contact Us
Dibenzoyl-L-tartaric acid

Product
Dibenzoyl-L-tartaric acid
CAS
2743-38-6
Formula
C18H14O8
Product Description
Dibenzoyl-L-tartaric acid is a chemical compound with the formula C18H14O8, commonly used in various chemical applications. It is known for its role as a chiral auxiliary in asymmetric synthesis, aiding in the production of enantiomerically pure compounds.
Need a quote for Dibenzoyl-L-tartaric acid?
Please fill out the RFQ form, and one of our representatives will contact you as soon as possible.
Typical Product Specifications
Canonicalized Compound
1
Compound Complexity
483
Hydrogen Bond Acceptor Count
8
Hydrogen Bond Donor Count
2
Rotatable Bond Count
9
Allowed IUPAC Name
(2R,3R)-2,3-dibenzoyloxybutanedioic acid
CAS-like Style IUPAC Name
(2R,3R)-2,3-dibenzoyloxybutanedioic acid
Markup IUPAC Name
(2<I>R</I>,3<I>R</I>)-2,3-dibenzoyloxybutanedioic acid
Preferred IUPAC Name
(2R,3R)-2,3-dibenzoyloxybutanedioic acid
Systematic IUPAC Name
(2R,3R)-2,3-bis(phenylcarbonyloxy)butanedioic acid
Traditional IUPAC Name
(2R,3R)-2,3-dibenzoyloxysuccinic acid
Standard InChI
InChI=1S/C18H14O8/c19-15(20)13(25-17(23)11-7-3-1-4-8-11)14(16(21)22)26-18(24)12-9-5-2-6-10-12/h1-10,13-14H,(H,19,20)(H,21,22)/t13-,14-/m1/s1
Standard InChIKey
YONLFQNRGZXBBF-ZIAGYGMSSA-N
XLogP3-AA Log P
2.6
Exact Mass
358.06886740
Molecular Weight
358.3
Canonical SMILES
C1=CC=C(C=C1)C(=O)OC(C(C(=O)O)OC(=O)C2=CC=CC=C2)C(=O)O
Isomeric SMILES
C1=CC=C(C=C1)C(=O)O[C@H]([C@H](C(=O)O)OC(=O)C2=CC=CC=C2)C(=O)O
Polar Surface Area Topological
127
MonoIsotopic Weight
358.06886740
Chemical Structure

Class
Uses of Dibenzoyl-L-tartaric acid
In the pharmaceutical and chemical industries, Dibenzoyl-L-tartaric acid is primarily utilized as a chiral auxiliary in the synthesis of pharmaceuticals and other fine chemicals. Its ability to influence the stereochemistry of reactions makes it valuable in the production of drugs that require specific enantiomeric forms.
Industries that use Dibenzoyl-L-tartaric acid
- Pharmaceutical - Used in the synthesis of chiral drugs, ensuring the production of the desired enantiomer for therapeutic efficacy.
- Chemical Manufacturing - Serves as a reagent in various chemical reactions, particularly in the production of fine chemicals and intermediates.
- Research and Development - Employed in laboratories for the development of new synthetic methodologies and in the study of stereochemistry.
Related Products
- Complementary Products
- Alternative Products
Contact Us


