L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]-

Get a Quick Quote Now
Select Packaging
Product
L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]-
CAS
143038-46-4
Formula
C27H32N2O6
Product Description
L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]- is a specialized amino acid derivative with a complex structure, often used in peptide synthesis and pharmaceutical applications. It features a unique combination of functional groups that enhance its reactivity and utility in various chemical processes.
Need a quote for L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]-?
Please fill out the RFQ form, and one of our representatives will contact you as soon as possible.
Typical Product Specifications
Canonicalized Compound
1
Compound Complexity
758
Hydrogen Bond Acceptor Count
6
Hydrogen Bond Donor Count
2
Rotatable Bond Count
9
Allowed IUPAC Name
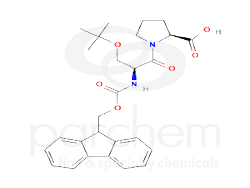
(2S)-1-[(2S)-3-tert-butoxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)propanoyl]pyrrolidine-2-carboxylic acid
Chemical Structure
Chemical Structure
CAS-like Style IUPAC Name
(2S)-1-[(2S)-2-[[9H-fluoren-9-ylmethoxy(oxo)methyl]amino]-3-[(2-methylpropan-2-yl)oxy]-1-oxopropyl]-2-pyrrolidinecarboxylic acid
Markup IUPAC Name
(2<I>S</I>)-1-[(2<I>S</I>)-2-(9<I>H</I>-fluoren-9-ylmethoxycarbonylamino)-3-[(2-methylpropan-2-yl)oxy]propanoyl]pyrrolidine-2-carboxylic acid
Preferred IUPAC Name
(2S)-1-[(2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-[(2-methylpropan-2-yl)oxy]propanoyl]pyrrolidine-2-carboxylic acid
Systematic IUPAC Name
(2S)-1-[(2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-[(2-methylpropan-2-yl)oxy]propanoyl]pyrrolidine-2-carboxylic acid
Traditional IUPAC Name
(2S)-1-[(2S)-3-tert-butoxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)propanoyl]proline
Standard InChI
InChI=1S/C27H32N2O6/c1-27(2,3)35-16-22(24(30)29-14-8-13-23(29)25(31)32)28-26(33)34-15-21-19-11-6-4-9-17(19)18-10-5-7-12-20(18)21/h4-7,9-12,21-23H,8,13-16H2,1-3H3,(H,28,33)(H,31,32)/t22-,23-/m0/s1
Standard InChIKey
ZEGKALAMRJSQJC-GOTSBHOMSA-N
XLogP3-AA Log P
3.6
Exact Mass
480.22603674
Molecular Weight
480.6
Canonical SMILES
CC(C)(C)OCC(C(=O)N1CCCC1C(=O)O)NC(=O)OCC2C3=CC=CC=C3C4=CC=CC=C24
Isomeric SMILES
CC(C)(C)OC[C@@H](C(=O)N1CCC[C@H]1C(=O)O)NC(=O)OCC2C3=CC=CC=C3C4=CC=CC=C24
Polar Surface Area Topological
105
MonoIsotopic Weight
480.22603674
Class
Uses of L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]-
This product is primarily used in the synthesis of peptides, as an intermediate in the production of pharmaceuticals. Its unique structure allows it to serve as a building block in drug development, particularly in the creation of biologically active compounds. Additionally, it may be utilized in research settings for the study of protein interactions and functions.
Industries that use L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]-
- Pharmaceuticals - In the pharmaceutical industry, this compound is used as an intermediate in the synthesis of active pharmaceutical ingredients (APIs) and in the development of new drugs.
- Biotechnology - Biotechnology companies utilize this amino acid derivative in research and development for creating novel peptides and studying their biological activities.
- Research and Development - In academic and industrial research settings, it is used for peptide synthesis and in various biochemical assays to explore protein functions.
Related Products
- Complementary Products
- Alternative Products
Contact Us
L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]-

Product
L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]-
CAS
143038-46-4
Formula
C27H32N2O6
Product Description
L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]- is a specialized amino acid derivative with a complex structure, often used in peptide synthesis and pharmaceutical applications. It features a unique combination of functional groups that enhance its reactivity and utility in various chemical processes.
Need a quote for L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]-?
Please fill out the RFQ form, and one of our representatives will contact you as soon as possible.
Typical Product Specifications
Canonicalized Compound
1
Compound Complexity
758
Hydrogen Bond Acceptor Count
6
Hydrogen Bond Donor Count
2
Rotatable Bond Count
9
Allowed IUPAC Name
(2S)-1-[(2S)-3-tert-butoxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)propanoyl]pyrrolidine-2-carboxylic acid
Chemical Structure

Chemical Structure

CAS-like Style IUPAC Name
(2S)-1-[(2S)-2-[[9H-fluoren-9-ylmethoxy(oxo)methyl]amino]-3-[(2-methylpropan-2-yl)oxy]-1-oxopropyl]-2-pyrrolidinecarboxylic acid
Markup IUPAC Name
(2<I>S</I>)-1-[(2<I>S</I>)-2-(9<I>H</I>-fluoren-9-ylmethoxycarbonylamino)-3-[(2-methylpropan-2-yl)oxy]propanoyl]pyrrolidine-2-carboxylic acid
Preferred IUPAC Name
(2S)-1-[(2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-[(2-methylpropan-2-yl)oxy]propanoyl]pyrrolidine-2-carboxylic acid
Systematic IUPAC Name
(2S)-1-[(2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-[(2-methylpropan-2-yl)oxy]propanoyl]pyrrolidine-2-carboxylic acid
Traditional IUPAC Name
(2S)-1-[(2S)-3-tert-butoxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)propanoyl]proline
Standard InChI
InChI=1S/C27H32N2O6/c1-27(2,3)35-16-22(24(30)29-14-8-13-23(29)25(31)32)28-26(33)34-15-21-19-11-6-4-9-17(19)18-10-5-7-12-20(18)21/h4-7,9-12,21-23H,8,13-16H2,1-3H3,(H,28,33)(H,31,32)/t22-,23-/m0/s1
Standard InChIKey
ZEGKALAMRJSQJC-GOTSBHOMSA-N
XLogP3-AA Log P
3.6
Exact Mass
480.22603674
Molecular Weight
480.6
Canonical SMILES
CC(C)(C)OCC(C(=O)N1CCCC1C(=O)O)NC(=O)OCC2C3=CC=CC=C3C4=CC=CC=C24
Isomeric SMILES
CC(C)(C)OC[C@@H](C(=O)N1CCC[C@H]1C(=O)O)NC(=O)OCC2C3=CC=CC=C3C4=CC=CC=C24
Polar Surface Area Topological
105
MonoIsotopic Weight
480.22603674
Class
Uses of L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]-
This product is primarily used in the synthesis of peptides, as an intermediate in the production of pharmaceuticals. Its unique structure allows it to serve as a building block in drug development, particularly in the creation of biologically active compounds. Additionally, it may be utilized in research settings for the study of protein interactions and functions.
Industries that use L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]-
- Pharmaceuticals - In the pharmaceutical industry, this compound is used as an intermediate in the synthesis of active pharmaceutical ingredients (APIs) and in the development of new drugs.
- Biotechnology - Biotechnology companies utilize this amino acid derivative in research and development for creating novel peptides and studying their biological activities.
- Research and Development - In academic and industrial research settings, it is used for peptide synthesis and in various biochemical assays to explore protein functions.
Related Products
- Complementary Products
- Alternative Products
Contact Us


