N,N-Dimethyloctanamide

Get a Quick Quote Now
Select Packaging
Product
N,N-Dimethyloctanamide
CAS
1118-92-9
Formula
C10H21NO
Product Description
L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]- is a specialized amino acid derivative with a complex structure, often used in peptide synthesis and pharmaceutical applications. It features a unique combination of functional groups that enhance its reactivity and utility in various chemical processes.
Need a quote for N,N-Dimethyloctanamide?
Please fill out the RFQ form, and one of our representatives will contact you as soon as possible.
Typical Product Specifications
Chemical Structure
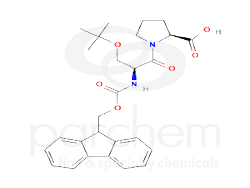
Canonicalized Compound
1
Compound Complexity
758
Hydrogen Bond Acceptor Count
6
Hydrogen Bond Donor Count
2
Rotatable Bond Count
9
Allowed IUPAC Name
(2S)-1-[(2S)-3-tert-butoxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)propanoyl]pyrrolidine-2-carboxylic acid
CAS-like Style IUPAC Name
(2S)-1-[(2S)-2-[[9H-fluoren-9-ylmethoxy(oxo)methyl]amino]-3-[(2-methylpropan-2-yl)oxy]-1-oxopropyl]-2-pyrrolidinecarboxylic acid
Markup IUPAC Name
(2<I>S</I>)-1-[(2<I>S</I>)-2-(9<I>H</I>-fluoren-9-ylmethoxycarbonylamino)-3-[(2-methylpropan-2-yl)oxy]propanoyl]pyrrolidine-2-carboxylic acid
Preferred IUPAC Name
(2S)-1-[(2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-[(2-methylpropan-2-yl)oxy]propanoyl]pyrrolidine-2-carboxylic acid
Systematic IUPAC Name
(2S)-1-[(2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-[(2-methylpropan-2-yl)oxy]propanoyl]pyrrolidine-2-carboxylic acid
Traditional IUPAC Name
(2S)-1-[(2S)-3-tert-butoxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)propanoyl]proline
Standard InChI
InChI=1S/C27H32N2O6/c1-27(2,3)35-16-22(24(30)29-14-8-13-23(29)25(31)32)28-26(33)34-15-21-19-11-6-4-9-17(19)18-10-5-7-12-20(18)21/h4-7,9-12,21-23H,8,13-16H2,1-3H3,(H,28,33)(H,31,32)/t22-,23-/m0/s1
Standard InChIKey
ZEGKALAMRJSQJC-GOTSBHOMSA-N
XLogP3-AA Log P
3.6
Exact Mass
480.22603674
Molecular Weight
480.6
Canonical SMILES
CC(C)(C)OCC(C(=O)N1CCCC1C(=O)O)NC(=O)OCC2C3=CC=CC=C3C4=CC=CC=C24
Isomeric SMILES
CC(C)(C)OC[C@@H](C(=O)N1CCC[C@H]1C(=O)O)NC(=O)OCC2C3=CC=CC=C3C4=CC=CC=C24
Polar Surface Area Topological
105
MonoIsotopic Weight
480.22603674
Class
Uses of N,N-Dimethyloctanamide
This product is primarily used in the synthesis of peptides, as an intermediate in the production of pharmaceuticals. Its unique structure allows it to serve as a building block in drug development, particularly in the creation of biologically active compounds. Additionally, it may be utilized in research settings for the study of protein interactions and functions.
Industries that use N,N-Dimethyloctanamide
- Pharmaceuticals - In the pharmaceutical industry, this compound is used as an intermediate in the synthesis of active pharmaceutical ingredients (APIs) and in the development of new therapeutic agents.
- Biotechnology - Biotechnology firms utilize this amino acid derivative in research and development for creating novel peptides and proteins, which can lead to advancements in drug discovery and therapeutic applications.
- Research and Development - In academic and industrial research settings, this compound is employed in studies related to protein chemistry and molecular biology, aiding in the understanding of protein structure and function.
Related Products
- Complementary Products
- Alternative Products
Contact Us
N,N-Dimethyloctanamide

Product
N,N-Dimethyloctanamide
CAS
1118-92-9
Formula
C10H21NO
Product Description
L-Proline,1-[O-(1,1-dimethylethyl)-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-seryl]- is a specialized amino acid derivative with a complex structure, often used in peptide synthesis and pharmaceutical applications. It features a unique combination of functional groups that enhance its reactivity and utility in various chemical processes.
Need a quote for N,N-Dimethyloctanamide?
Please fill out the RFQ form, and one of our representatives will contact you as soon as possible.
Typical Product Specifications
Chemical Structure

Canonicalized Compound
1
Compound Complexity
758
Hydrogen Bond Acceptor Count
6
Hydrogen Bond Donor Count
2
Rotatable Bond Count
9
Allowed IUPAC Name
(2S)-1-[(2S)-3-tert-butoxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)propanoyl]pyrrolidine-2-carboxylic acid
CAS-like Style IUPAC Name
(2S)-1-[(2S)-2-[[9H-fluoren-9-ylmethoxy(oxo)methyl]amino]-3-[(2-methylpropan-2-yl)oxy]-1-oxopropyl]-2-pyrrolidinecarboxylic acid
Markup IUPAC Name
(2<I>S</I>)-1-[(2<I>S</I>)-2-(9<I>H</I>-fluoren-9-ylmethoxycarbonylamino)-3-[(2-methylpropan-2-yl)oxy]propanoyl]pyrrolidine-2-carboxylic acid
Preferred IUPAC Name
(2S)-1-[(2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-[(2-methylpropan-2-yl)oxy]propanoyl]pyrrolidine-2-carboxylic acid
Systematic IUPAC Name
(2S)-1-[(2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-[(2-methylpropan-2-yl)oxy]propanoyl]pyrrolidine-2-carboxylic acid
Traditional IUPAC Name
(2S)-1-[(2S)-3-tert-butoxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)propanoyl]proline
Standard InChI
InChI=1S/C27H32N2O6/c1-27(2,3)35-16-22(24(30)29-14-8-13-23(29)25(31)32)28-26(33)34-15-21-19-11-6-4-9-17(19)18-10-5-7-12-20(18)21/h4-7,9-12,21-23H,8,13-16H2,1-3H3,(H,28,33)(H,31,32)/t22-,23-/m0/s1
Standard InChIKey
ZEGKALAMRJSQJC-GOTSBHOMSA-N
XLogP3-AA Log P
3.6
Exact Mass
480.22603674
Molecular Weight
480.6
Canonical SMILES
CC(C)(C)OCC(C(=O)N1CCCC1C(=O)O)NC(=O)OCC2C3=CC=CC=C3C4=CC=CC=C24
Isomeric SMILES
CC(C)(C)OC[C@@H](C(=O)N1CCC[C@H]1C(=O)O)NC(=O)OCC2C3=CC=CC=C3C4=CC=CC=C24
Polar Surface Area Topological
105
MonoIsotopic Weight
480.22603674
Class
Uses of N,N-Dimethyloctanamide
This product is primarily used in the synthesis of peptides, as an intermediate in the production of pharmaceuticals. Its unique structure allows it to serve as a building block in drug development, particularly in the creation of biologically active compounds. Additionally, it may be utilized in research settings for the study of protein interactions and functions.
Industries that use N,N-Dimethyloctanamide
- Pharmaceuticals - In the pharmaceutical industry, this compound is used as an intermediate in the synthesis of active pharmaceutical ingredients (APIs) and in the development of new therapeutic agents.
- Biotechnology - Biotechnology firms utilize this amino acid derivative in research and development for creating novel peptides and proteins, which can lead to advancements in drug discovery and therapeutic applications.
- Research and Development - In academic and industrial research settings, this compound is employed in studies related to protein chemistry and molecular biology, aiding in the understanding of protein structure and function.
Related Products
- Complementary Products
- Alternative Products
Contact Us


