Salmeterol EP Impurity G

Get a Quick Quote Now
Select Packaging
Product Description
Product
Salmeterol EP Impurity G
CAS
1391051-88-9
Formula
C50H72N2O7
Synonym
Salmeterol EP Impurity G, 1391051-88-9, 2-[[[2-Hydroxy-2-[4-hydroxy-3-(hydroxymethyl)phenyl]ethyl]-[6-(4-phenylbutoxy)hexyl]amino]methyl]-5-[1-hydroxy-2-[6-(4-phenylbutoxy)hexylamino]ethyl]phenol, Salmeterol Impurity G, BCP25657, AKOS027327317, 4-(1-Hydroxy-2-((2-hydroxy-4-(1-hydroxy-2-((6-(4-phenylbutoxy)hexyl)amino)ethyl)benzyl)(6-(4-phenylbutoxy)hexyl)amino)ethyl)-2-(hydroxymethyl)phenol, FT-0701237
Typical Product Specifications
Chemical Structure
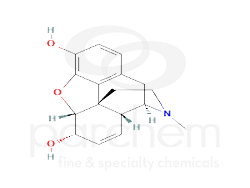
Class
Salmeterol EP Impurity G

Product Description
Product
Salmeterol EP Impurity G
CAS
1391051-88-9
Formula
C50H72N2O7
Synonym
Salmeterol EP Impurity G, 1391051-88-9, 2-[[[2-Hydroxy-2-[4-hydroxy-3-(hydroxymethyl)phenyl]ethyl]-[6-(4-phenylbutoxy)hexyl]amino]methyl]-5-[1-hydroxy-2-[6-(4-phenylbutoxy)hexylamino]ethyl]phenol, Salmeterol Impurity G, BCP25657, AKOS027327317, 4-(1-Hydroxy-2-((2-hydroxy-4-(1-hydroxy-2-((6-(4-phenylbutoxy)hexyl)amino)ethyl)benzyl)(6-(4-phenylbutoxy)hexyl)amino)ethyl)-2-(hydroxymethyl)phenol, FT-0701237
Typical Product Specifications
Chemical Structure

Class


