Gadoversetamide

Get a Quick Quote Now
Select Packaging
Product Description
Product
Gadoversetamide
CAS
131069-91-5
Formula
C20H34N5O10
Synonym
GADOVERSETAMIDE, Optimark, 131069-91-5, RLM74T3Z9D, MP-1177, (8,11-bis(carboxymethyl)-14-(2-((2-methoxyethyl)amino)-2-oxoethyl)-6-oxo-2-oxa-5,8,11,14-tetraazahexadecan-16-oato(3-)), gadolinium, Optimark (TN), [N,N-Bis[2-[(carboxymethyl)[[(2-methoxyethyl)carbamoyl]methyl]amino]ethyl]glycinato(3-)]gadolinium, GADOVERSETAMIDE [MI], GADOVERSETAMIDE [INN], GADOVERSETAMIDE [JAN], GADOVERSETAMIDE [HSDB], GADOVERSETAMIDE [USAN], SCHEMBL237265, GADOVERSETAMIDE [VANDF], GADOVERSETAMIDE [MART.], GADOVERSETAMIDE [USP-RS], GADOVERSETAMIDE [WHO-DD], CHEBI:31644, Gadoversetamide (JAN/USP/INN), GADOVERSETAMIDE [EMA EPAR], EX-A5524, GADOVERSETAMIDE [ORANGE BOOK], GADOVERSETAMIDE [USP IMPURITY], AKOS015896615, DB00538, GADOVERSETAMIDE [USP MONOGRAPH], D01646, Q5516432, gadolinium 8,11-bis(carboxylatomethyl)-14-{2-[(2-methoxyethyl)amino]-2-oxoethyl}-6-oxo-2-oxa-5,8,11,14-tetraazahexadecan-16-oate
Typical Product Specifications
Molecular weight
661.77
Assay/Purity
Typically NLT 98%
Chemical Structure
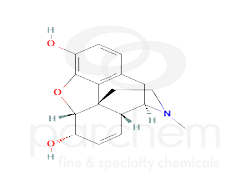
Notes
Gadoversetamide is classified as an: Active Pharmaceutical Ingredient (API) / Pharmaceutical Intermediate
Class
Industry
Gadoversetamide

Product Description
Product
Gadoversetamide
CAS
131069-91-5
Formula
C20H34N5O10
Synonym
GADOVERSETAMIDE, Optimark, 131069-91-5, RLM74T3Z9D, MP-1177, (8,11-bis(carboxymethyl)-14-(2-((2-methoxyethyl)amino)-2-oxoethyl)-6-oxo-2-oxa-5,8,11,14-tetraazahexadecan-16-oato(3-)), gadolinium, Optimark (TN), [N,N-Bis[2-[(carboxymethyl)[[(2-methoxyethyl)carbamoyl]methyl]amino]ethyl]glycinato(3-)]gadolinium, GADOVERSETAMIDE [MI], GADOVERSETAMIDE [INN], GADOVERSETAMIDE [JAN], GADOVERSETAMIDE [HSDB], GADOVERSETAMIDE [USAN], SCHEMBL237265, GADOVERSETAMIDE [VANDF], GADOVERSETAMIDE [MART.], GADOVERSETAMIDE [USP-RS], GADOVERSETAMIDE [WHO-DD], CHEBI:31644, Gadoversetamide (JAN/USP/INN), GADOVERSETAMIDE [EMA EPAR], EX-A5524, GADOVERSETAMIDE [ORANGE BOOK], GADOVERSETAMIDE [USP IMPURITY], AKOS015896615, DB00538, GADOVERSETAMIDE [USP MONOGRAPH], D01646, Q5516432, gadolinium 8,11-bis(carboxylatomethyl)-14-{2-[(2-methoxyethyl)amino]-2-oxoethyl}-6-oxo-2-oxa-5,8,11,14-tetraazahexadecan-16-oate
Typical Product Specifications
Molecular weight
661.77
Assay/Purity
Typically NLT 98%
Chemical Structure

Notes
Gadoversetamide is classified as an: Active Pharmaceutical Ingredient (API) / Pharmaceutical Intermediate
Class
Industry


